- Formula: HNaO
- Molecular weight: 39.9971
- IUPAC Standard InChI:
- InChI=1S/Na.H2O/h;1H2/q+1;/p-1
- Download the identifier in a file.
- IUPAC Standard InChIKey:HEMHJVSKTPXQMS-UHFFFAOYSA-M
- CAS Registry Number: 1310-73-2
- Chemical structure:
This structure is also available as a 2d Mol fileor as a computed3d SD file
The 3d structure may be viewed usingJavaorJavascript. - Species with the same structure:
- Information on this page:
- Other data available:
- Data at other public NIST sites:
- Options:
Data at NIST subscription sites:
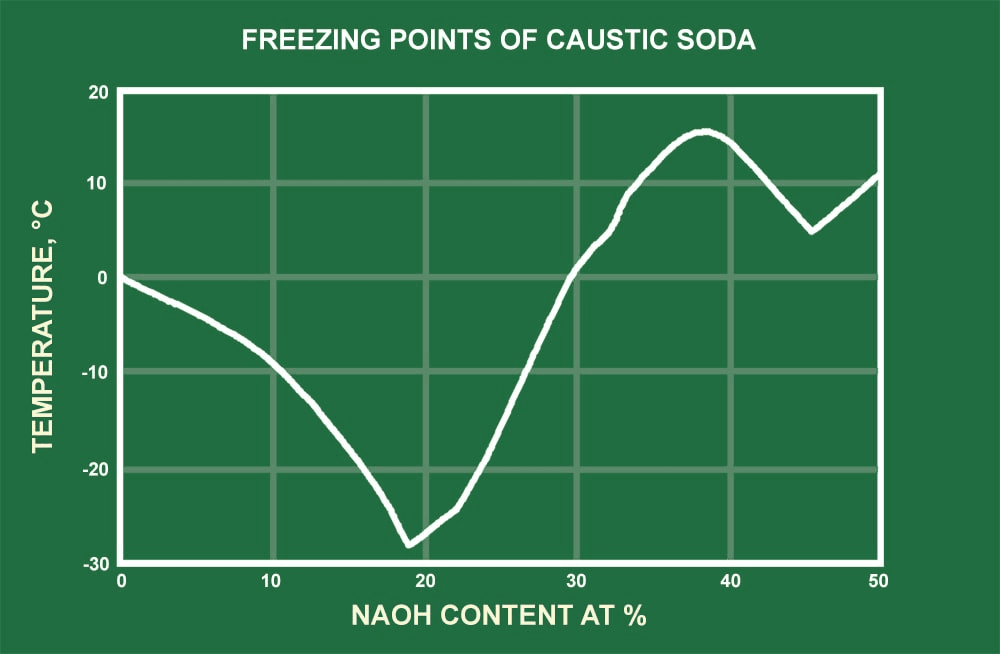
Table 3 Specific heats of caustic soda in Btu/lb°F 30 Graph 1 Boiling and solidifying temperature of aqueous caustic soda solutions 31 Graph 2 Specific gravity of aqueous caustic soda solutions 32. Determination of sodium hydroxide in caustic soda 46 Determination of sodium carbonate in caustic soda 49 Determination of sodium chloride in. It also reacts with weak-acid gases, such as hydrogen sulfide, sulfur dioxide, and carbon dioxide. Caustic soda reacts with amphoteric metals (Al, Zn, Sn) and their oxides to form complex anions such as AlO2(-), ZnO2(-2), SNO2(-2), and H2 (or H2O with oxides). All organic acids also react with sodium hydroxide to form soluble salts.
NIST subscription sites provide data under theNIST Standard ReferenceData Program, but require an annual fee to access.The purpose of the fee is to recover costs associatedwith the development of data collections included insuch sites. Your institution may already be a subscriber.Follow the links above to find out more about the datain these sites and their terms of usage.
Solid Phase Heat Capacity (Shomate Equation)
Go To:Top, References, Notes
Data compilation copyrightby the U.S. Secretary of Commerce on behalf of the U.S.A.All rights reserved.
Cp° = A + B*t + C*t2 + D*t3 + E/t2
H° − H°298.15= A*t + B*t2/2 + C*t3/3 + D*t4/4 − E/t + F − H
S° = A*ln(t) + B*t + C*t2/2 + D*t3/3 − E/(2*t2) + G
Cp = heat capacity (J/mol*K)
H° = standard enthalpy (kJ/mol)
S° = standard entropy (J/mol*K)
t = temperature (K) / 1000.
View plotRequires a JavaScript / HTML 5 canvas capable browser.
| Temperature (K) | 298. - 572. | 572. - 596. |
|---|---|---|
| A | 419.4837 | 86.02304 |
| B | -1717.754 | 0.000000 |
| C | 2953.573 | 0.000000 |
| D | -1597.221 | 0.000000 |
| E | -6.046884 | 0.000000 |
| F | -517.8662 | -448.8512 |
| G | 933.0738 | 169.6281 |
| H | -425.9312 | -425.9312 |
| Reference | Chase, 1998 | Chase, 1998 |
| Comment | Data last reviewed in December, 1970 | Data last reviewed in December, 1970 |
| Temperature (K) | Cp (J/mol*K) | S° (J/mol*K) | -(G° - H°298.15)/T (J/mol*K) | H° - H°298.15 (kJ/mol) |
|---|---|---|---|---|
| 298. | 59.52 | 64.43 | 64.44 | -0.00 |
| 300. | 59.67 | 64.83 | 64.45 | 0.12 |
| 400. | 64.94 | 82.71 | 66.85 | 6.34 |
| 500. | 75.16 | 98.17 | 71.59 | 13.29 |
| Temperature (K) | Cp (J/mol*K) | S° (J/mol*K) | -(G° - H°298.15)/T (J/mol*K) | H° - H°298.15 (kJ/mol) |
|---|---|---|---|---|
| 572. | 86.02 | 121.6 | 75.62 | 26.29 |
References
Go To:Top, Solid Phase Heat Capacity (Shomate Equation), Notes
Data compilation copyrightby the U.S. Secretary of Commerce on behalf of the U.S.A.All rights reserved.
Chase, 1998
Chase, M.W., Jr.,NIST-JANAF Themochemical Tables, Fourth Edition,J. Phys. Chem. Ref. Data, Monograph 9, 1998, 1-1951. [all data]
Notes
Go To:Top, Solid Phase Heat Capacity (Shomate Equation), References
- Data from NIST Standard Reference Database 69:NIST Chemistry WebBook
- The National Institute of Standards and Technology (NIST)uses its best efforts to deliver a high quality copy of theDatabase and to verify that the data contained therein havebeen selected on the basis of sound scientific judgment.However, NIST makes no warranties to that effect, and NISTshall not be liable for any damage that may result fromerrors or omissions in the Database.
- Customer supportfor NIST Standard Reference Data products.
Click to see full answer.
Similarly, what is the heat of neutralization of HCl and NaOH?
The heat of reaction of one mole of H+ and OH- is 57.3 KJ. So, the heat of neutralisation of HCl and NaOH will be very cery close to 57.3 KJ per mole( As Both HCl and NaOH are strong elctrolytes so both of them quite easily without any considerable expense of energy furnish H+ and OH- ions respectively.

Specific Heat Capacity Bbc Bitesize
why is the reaction between NaOH and HCl exothermic? - When a reaction is endothermic - Bonds are broken and energy is absorbed from the surroundings. In your example of HCl + NaOH - this is a neutralisation reaction to form NaCl + H20. Basically there is more bond making than bond breaking in this reaction so the Delta H is negative - it is more exothermic.
In this manner, what equation is appropriate to calculate the heat produced from the HCl NaOH reaction?
Calculate the number of moles of base you add to determine the molar heat of neutralization, expressed using the equation ΔH = Q ÷ n, where 'n' is the number of moles. For example, suppose you add 25 mL of 1.0 M NaOH to your HCl to produce a heat of neutralization of 447.78 Joules.
Specific Heat Capacity Of Water
Is the reaction between HCl and NaOH endothermic or exothermic?
Specific Heat Capacity Calculator
This reaction is classified as an exothermic reaction. The reaction of HCl(aq), a strong acid, with NaOH(aq), a strong base, is an exothermic reaction.